Problems in Brooding
Aspergillosis (Fungal Pneumonia)
Aspegillosis is the name of a fungus that can kill game birds. Birds can breathe in spores if exposed for example to aspergillus spores in the hatchery following pipping, or if exposed to spores in the brooding facilities (in mouldy straw or savings)
Spores can be derived form many sources but common sources include litter and feed. Only use first quality fresh clean litter and ensure damp litter is removed. A source of fresh feed that has been stored in optimal conditions should also be available. Aspergillus moulds can be seen in feed or litter such as straw as a blue or black dusty mould.
Poults may lose weight and show gasping with the neck outstretched as described with gapes infection. These birds often die within a few days. Significant levels of mortality can be observed if fungal spore contamination is high.
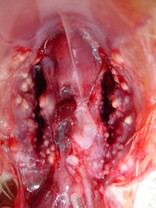
Post mortem examination can reveal hard white to grey nodules throughout the lung fields and within the airsacs of birds. Occasionally cheesy plaques can form in the windpipe occluding it resulting in asphyxia.
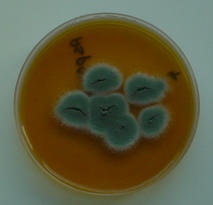
Swabs can be taken from the lesions at the time of post mortem. These can be cultured using techniques to grow fungi. The most common species affecting birds is Aspergillus fumigatus.
There is no treatment for Aspergillus and affected poults require culling on welfare grounds.Improving egg hygiene, hatchery hygiene, brooding ventilation and using first quality litter and feed can reduce the risk.
Non starters (starve outs)
This is a common diagnosis for high early mortality in poults in the first 7 days of life. Poults can often look well when they are placed in the brooding facility but then mortality develops within 48 hours and can persist to 7 days of age. Birds become weak and lethargic and can be seen hunched up or huddling. Further examinations often reveals these birds have failed to feed or consumed litter material resulting in impactions.
Just before pipping the embryo starts to absorb the yolk from within the egg. This yolk sac becomes the main source of food and energy for the bird in the first three to four days of life. During these first few days the bird will absorb the yolk sac contents into the intestine where it is then utilised by the bird. During this time it is paramount that the bird then learns to feed and drink and settles into its surroundings. If the bird fails to feed or drink by the time the reserve of yolk sac is depleted then it will lose its source of energy and then become weak, lethargic and then completely disinterested in feedigg or drinking. Such birds will then develop dehydration kidney damage and death.
The causes of non starters are complex. Any stress that can result in a reduced ability for the birds to eat and drink can act as a potential cause. If chicks are derived from eggs from a parent flock that is young, has a disease problem or nutritional issue this could result in weak chicks. Secondly if there have been any problems in the incubation period with temperature, humidity or ventilation then poult viability may be affected. This can sometimes happens when there is a protracted hatch ie when there is a long time period between the first and last poult hatching. This then means that some birds may be in the hatcher for an extended amount of time during which there yolk sac reserves are becoming depleted and they are becoming dehydrated.
Following take off if there is any period during which poults are chilled then this can lead to significant losses during brooding. This could be in the holding room or during transportation or following placement in the brooding facility. Poults should not be held in the hatchery where ever possible and optimal temperature control facilities should be available in this environment and during transportation. These facilities should be free from draughts and have adequate ventilation.
Brooding facilities should have been fully prepared and the environmental conditions primed at least 24 hours before the planned arrival of poults. Floor temperatures are more critical than air temperatures. Brooders should be set to allow birds a range of temperatures so that individuals can find the temperature they are most suited too. Feed availability is critical and a good quality fresh source of feed should be available at all times. Water should be cold, fresh and also easily accessible to the birds. Litter should be of an appropriate substrate to allow good floor insulation, good mobility and easily distinguishable from the feed source. Lighting needs to be of an appropriate level to allow birds to easily find feed and water. Where gas brooders are being used ventilation is even more critical to prevent the build up of toxic gases.
Occasionally birds may present as non starters with failure to feed as a result of a disease process even if all management factors are optimal. Examples include bacterial infections such as Salmonella infection and viral infections such as rotavirus. The provision of multivitamins in the water can help to give the birds a boost in the post hatch period. Some supplements also include glucose for energy as well as electrolytes.

Yolk sac infection (Omphalitis)
Yolk sac infection can be a common cause of losses. The yolk sac is absorbed just before hatching. This is critical for the birds survival in the immediate post hatch period as a source of nutrition, energy and antibodies.Good hygiene is always critical to prevent yolk sac infection. Infection can take place at a number of different times in the life of the egg and poult.
The first point that contamination can take place is immediately after the egg has been laid before the cutical has dried. This leaves the porous egg shell open to contamination. It is therefore important to ensure ample well managed nests to prevent floor eggs. Maintaining nests with optimal hygiene with regular egg collection is also critical to reduce the incidence of contamination. The nutrition and management of breeders requires great attention to detail to ensure optimal egg and egg shell quality. Poor shell quality can predispose eggs to contamination even in relatively hygienic conditions.
Contamination can also take place following egg collection. Handling of hatching eggs should be regular and with care. Storage of eggs should be well managed to ensure condensation of the egg shells does not occur. A dedicated egg store with temperature and humidity control should be the standard. Egg storage times should be kept to a minimum and where possible not exceed 7 days.
Careful consideration should be given to whether there is a need to wash eggs. Floor eggs should ideally not be set at all as they can act as an infection reservoir for the setter and hatcher. If eggs are washed then procedures must be followed carefully. The correct temperature of wash water, exposure times, and sanitiser needs to be used. Regular replenishing of sanitiser in accordance with the manufacturers guidelines is critical to prevent contamination of eggs. Floor eggs and washed eggs should be set at the bottom of the setter. Consideration for some form of sanitisation of eggs should be given.
It is critical to ensure that all setter and hatchers have had through cleansing and disinfection before placing eggs. Disinfectants should be DEFRA approved and used in accordance with General Orders rate. The choice of disinfection is critical and it should not be detrimental to the equipment within the setter and the hatchers.
Once eggs are in the setters it is useful to candle and remove infertile eggs or early dead at around 14 days of age. These eggs if contaminated can result in bangers and further contamination of clean eggs within the setters. In some systems eggs require movement from the setters to the hatchers using transfer machines. It is again critical that the hygiene of these machines is optimal. Following transfer to the setters it is possible to fumigate the eggs at pipping to help to reduce contamination. This should be done observing operator health and safety concerns particularly if using a product such as formaldehyde.
Once the poults have hatched the yolk sac can still be contaminated. This can happen when the navals of poults are poorly healed. In this situation bacteria can track into the yolk sac resulting in yolk sac infection. Poorly healed navals can result if incubation has not been optimal or birds have been taken out of the hatchers too early.
Yolk sac infection can sometimes be detected by a bad smell in the brooder unit (similar to rotten eggs). This can often result in dead on arrival poults at the brooding facilty, early dead in good sized chicks, and losses even when poults have fed.
There are a number of potential bacteria that may be involved but this can range from E.coli, Pseudomonas, Enterococcus and Salmonella. Sometimes more specific bacteria can be identified. It is important that a post mortem examination is conducted to identify firstly whether the cause of mortality is as a result of yolk sac infection and secondly to determine what bacteria is causing the mortality. It is then possible to determine what antibiotic treatment would be appropriate. Often treatment of yolk sac infection can be effective in reducing mortality but often these birds have chronic damage internally which then results in stunted birds which fail to compete.
Salmonella infection
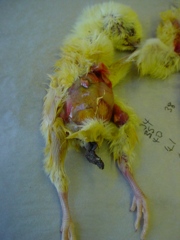
Acute mortality in poults can sometimes be associated with Salmonella infection. The losses can often be very high from a very young age. Morbidity can also be high with a high percentage of lethargic birds observed. There are a number of salmonella species that can be involved including Salmonella binza (orion), Salmonella typhimurium, Salmonella enteritidis and Salmonella pullorum. Salmonella infections can be from contamination at any period in the hatchery but recycling can occur within the hatchery and in brooding facilities. Breeding stock can also act as a source of infection. It is possible for infection to pass directly through the eggs from the breeding birds.
Antibiotic treatment can be used but is often discouraged due to public health concerns regarding antibiotic resistance with agents such as Salmonella typhimurium. It is therefore important to source poults from clean breeding stock, a clean hatchery and high standards of cleansing and disinfection should be maintained on the brooding site. Post mortem often reveals little in the way of gross pathology but occasionally liver congestion and cheesy cores can be observed in the caeca (last part of intestine). Salmonella infection can be confirmed by a veterinary laboratory using microbiological techniques.
Viral enteritis - ROTAVIRUS
Viral enteritis is a term to describe a viral infection that targets the gut of the bird. These viruses can cause very severe damage to young poults from the age of 3 to 4 days. Very high mortalities of up to 50-60% can be observed where heavy challenges take place. Flocks often present with a large number of birds huddling and described as "sweating". The birds are often lethargic and they will fail to feed and then die in large numbers within 4-7 days of infection. Occasionally birds will show signs of diarrhoea. Pasting of the vent region can also be observed.
It is possible that if poults show symptoms at a very young age that contamination has occurred shortly following hatching when chicks may be exposed to contaminated eggs shells.
The post mortem picture is often one of poor birds, with little muscle, failing to feed with an accumulation of yellow frothy material in the caeca (last part of the intestine). The caecae can be enlarged and the intestine can be thin walled with watery clear contents. Confirmatory diagnosis cannot be made from a post mortem alone. Further tests such as virus isolation, polyacrylamide gel electrophoresis, electronmicroscopy or DNA techniques can be used.
There is no specific treatment for Rotavirus but changes in management can help to reduce losses. Ensuring the birds have glucose supplementation, electrolytes and multivitamins via the drinking water can help to reduce losses. The use of probiotics may also be of benefit in helping to re-establish a normal gut flora. Further more increasing temperatures can prevent huddling and smothering. Fresh feed of an appropriate physical form must also be provided at all times. The use of antibiotics can also be of benefit where secondary bacterial overgrowth of the gut is suspected.
The key to rotavirus control is prevention. Rotavirus problems can persist on sites and infect poults from one season to the next. Therefore it is critical that a thorough cleansing and disinfection programme takes place at the end of the season and again at the start of the next season. The use of new equipment is preferable. Where possible new ground should be used each season to prevent carry over and any mobile housing also requires thorough cleansing and disinfection. Multi-age sites should be avoided whereever possible. If rotavirus establishes itself on a premises and biosecurity is not optimal then infection spreads between flocks. As the season progresses the viral load can built up on a site affecting birds at an even younger age. The younger the birds are when infected the poorer the prognosis. Following rota virus infection it is not uncommon for a flock to have poor uniformity and stunting.
No vaccine is available for rotavirus in gamebirds.
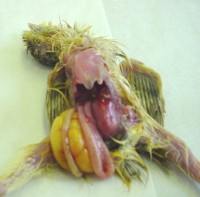
fluid in caeca




